What is chemical-looping combustion?
Chemical looping combustion is a combustion process where a direct contact between the fuel and the combustion air is avoided. This is accomplished by an oxygen carrrier, i.e. a metal oxide, by which the oxygen is transferred from the combustion air to the fuel. The major advantage is that carbon dioxide can be obtained in a separate stream without an energy-consuming separation process.
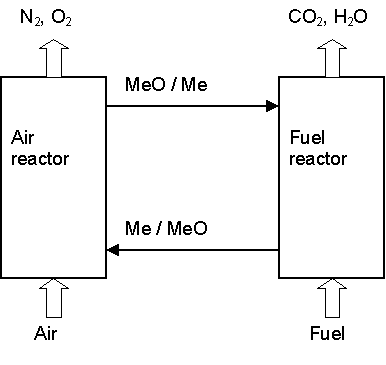
The heart of the system is an air and a fuel reactor, Fig. 1. The gaseous fuel, e.g. fossil gas, is introduced to the fuel reactor, where it is oxidised by the oxygen carrier, i.e. the metal oxide, MeO:
MeO + CH4 ® Me + 2H2O + CO2 (1)
The exit gas stream from the fuel reactor contains CO2 and H2O, and almost pure CO2 is obtained when H2O is condensed. The particles of the oxygen carrier are transferred to the air reactor where they are regenerated by taking up oxygen from the air:
Me + ŻO2 ® MeO (2)

Fig. 1. Chemical-looping combustion. MeO/Me denote recirculating oxygen-carrier particles. (A full conversion from MeO to Me and back to MeO, is not needed in the reactors.)
The air oxidizing the metal gives a flue gas containing only N2 and some unused O2. The total amount of heat evolved from reaction (1) and (2) is the same as for normal combustion, where the oxygen is in direct contact with the fuel. The significant advantage compared to normal combustion is that CO2 is not diluted with N2 but obtained pure without any energy needed for separation.
Technologies are available for storage of carbon dioxide at low costs, but the problem is the high costs for retrieval of carbon dioxide from the combustion process. A number of processes for carbon dioxide separation in connection with power production have been proposed in the literature1-5. The integration of CO2 capture in the power process, results in a decrease in efficiency of around 10 percentage units and the estimated cost increase is typically about 50% or higher1,6.
Chemical-looping combustion was first proposed as a means to enhance the thermal efficiency of power production7,8, but later the possibility to use this combustion process for CO2 separation without energy losses was recognised9,10. The idea is to use chemical-looping combustion instead of conventional combustion in power plants. In order to achieve a high thermal efficiency chemical-looping combustion should be integrated in a combined gas-turbine steam-power process. This also means that the process will be pressurized, which will allow for a compact design of the reactor system. For a first application the fuel is probably fossil gas, but gas from gasified solid fuels can also be used.
The important advantages of chemical-looping combustion are that the separation can be accomplished without energy losses and that gas cleaning devices, for instance in the form of scrubbers can be avoided.
Reactor design. There is little published on the lay-out of the reactor system, but Lyngfelt et al.11 proposed a circulating system based on two interconnected fluidised beds, a high velocity riser and a bubbling fluidised bed, Fig. 2. The bed material circulating between the two fluidised beds is the oxygen carrier in the form of metal oxide particles. In the air reactor, or the riser, oxygen is transferred from the combustion air to the oxygen carrier. In the bubbling fluidised bed, or the fuel reactor, oxygen is transferred from the oxygen carrier to the fuel. The gas volume flow in the air reactor is approximately ten times larger than that of the gaseous fuel, and to keep a reasonable size of the reactors a high velocity is chosen in the air reactor.
The gas velocity in the riser, provides the driving force for the circulation of particles between the two beds. Thus, the particles carried away from the riser are recovered by a cyclone and led to the fuel reactor. From the fuel reactor the particles are returned to the air reactor by means of gravity; the fuel reactor is located at a sufficiently high level.
After condensation of the water, the remaining gas, containing mostly CO2, is compressed and cooled in stages to yield liquid CO2. Remaining non-condensible gas from this stream, such as unreacted methane, is recycled to the fuel reactor. In order to avoid accumulation of noncombustible gases, such as N2, in the recycling loop, a minor part of this flow is bled to the air reactor.
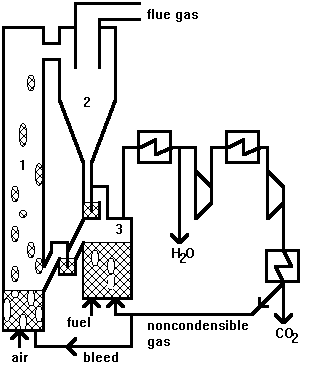
Fig. 2. Layout of chemical-looping combustion process, with two interconnected fluidised beds.
1) air reactor; 2) cyclone; 3) fuel reactor
Oxygen carriers.
The metal-oxide particles, used as an oxygen carrier in chemical-looping combustion, must have sufficient rates of reduction and oxidation, and possess enough strength to limit particle breakage and attrition. It is also an advantage if the particles are inexpensive and environmentally sound. A number of metals and their corresponding oxides have been discussed like Fe, Ni, Co, Cu, Mn and Cd, but experimental studies of oxygen carriers for this application are limited to Ni, Fe and Co 9,12-19. In order to increase both the reactivity and durability of the oxides, the particles have often been doped with Al2O3, yttria-stabilised zirconium (YSZ), TiO2 or MgO. Most of the studies were made with Ni.The process of chemical-looping combustion has also been demonstrated in a laboratory setup at a temperature of 1200░ C, with particles of NiO and Al2O317,20. The experimental work cited so far was made in Japan, but recently work with Fe has been made at Chalmers21,22. DOE also supports research with focus on Fe and Cu but no publications are yet available.
The Japanese data on oxygen carriers in combination with the design calculations11, show that there are oxygen carriers with suitable properties for such a process. Here the obvious advantage with fluidised beds should be pointed out, i.e. the large mass mass of particles which is in close contact with the gas. Therefore even relatively slow conversion rates, e.g. a few %/minute, are sufficient for a realistic process,11 which can be compared to rates experimentally obtained of up to 100-200 %/minute. The nickel oxide particles generally showed the highest reactivity. The reactivity of iron oxide particles, however, should be sufficient21,22, but other properties like resistance to attrition have to be improved for these particles.
____________________________1. Göttlicher G and Pruschek R Analysis of development potentials for power stations with CO2 removal/concentration. Proceedings of the 4th International Conference on Greenhouse Gas Control Technologies, Interlaken, 1998,83-88.
2. Simbeck D A portfolio selection approach for power plant CO2 capture, separation and R&D options. Proceedings of the 4th International Conference on Greenhouse Gas Control Technologies, Interlaken, 1998,119-124.
3. Chiesa P, Consonni S and Lozza G A comparative analysis of IGCCs with CO2 sequestration. Proceedings of the 4th International Conference on Greenhouse Gas Control Technologies, Interlaken, 1998,107-112.
4. Audus H, Kaarstad O and Skinner G CO2 capture by pre-combustion decarbonisation of natural gas. Proceedings of the 4th International Conference on Greenhouse Gas Control Technologies, Interlaken, 1998,557-562.
5. Bolland O and Undrum H Removal of CO2 from gas turbine power plants: evaluation of pre- and postcombustion methods. Proceedings of the 4th International Conference on Greenhouse Gas Control Technologies, Interlaken, 1998,125-130.
6. Lyngfelt, A. and Leckner, B. Technologies for CO2 separation. Minisymposium on Carbon Dioxide Capture and Storage, School of Environmental Sciences, Chalmers University of Technology and Göteborg University, Göteborg, October 22, 1999, pp. 25-35. (available on http://www.entek.chalmers.se/~anly/symp/sympco2.html)
7. Richter H and Knoche K Reversibility of combustion processes. ACS Symp. Ser, 1983, 235, 71-86.
8. Ishida M, Zheng D and Akehata T Evaluation of a chemical-looping-combustion power-generation system by graphic exergy analysis. Energy - the International Journal, 1987, 12, 147-154.
9. Ishida M and Jin H A new advanced power-generation system using chemical looping combustion. Energy, 1994, 19, 415-422.
10. Anheden M, Näsholm A-S and Svedberg G Chemical-looping combustion - efficient conversion of chemical energy in fuels into work. Proc. Intersoc. Energy Conversion Eng. Conf. 1995, 30, 75-81.
11. Lyngfelt A, Leckner B and Mattisson T A fluidized-bed combustion process with inherent CO2 separation - application of chemical-looping combustion. submitted for publication, 2000,
12. Nakano Y, Iwamoto S, Maeda T, Ishida M and Akehata T Characteristics of reduction and oxidization cyclic process by use of aFe2O3 medium. Iron & Steel Journal of Japan, 1986, 72, 1521-1527.
13. Ishida M and Jin H Novel chemical-looping combustor without NOx formation. Ind. Eng. Chem. Res. 1996, 35, 2469-2472.
14. Ishida M, Jin H and Okamoto T A fundamental study of a new kind of medium material for chemical-looping combustion. Energy & Fuels, 1996, 10, 958-963.
15. Ishida M, Jin H and Okamoto T Kinetic behaviour of solid particle in chemical-looping combustion: suppressing carbon deposition in reduction. Energy & Fuels, 1998, 12, 223-229.
16. Jin H, Okamoto T and Ishida M Development of a novel chemical-looping combustion: synthesis of a looping material with a double metal oxide of CoO-NiO. Energy & Fuels, 1998, 12, 1272-1277.
17. Ishida M, Yamamoto M and Saito Y Experimental works on innovative chemical-looping combustor. ECOS'99, International Conference on Efficiency, Costs, Optimization, Simulation and Environmental Aspects of Energy Systems, Tokyo, June 8-10, , 1999,306-310.
18. Jin H, Okamoto T and Ishida M Development of a novel chemical-looping combustion: synthesis of a solid looping material of NiO/NiAl2O4. Ind. Eng. Chem. Res, 1999, 38, 126-132.
19. Hatanaka T, Matsuda S and Hatano H A new-concept gas-solid combustion system "MERIT" for high combustion efficiency and low emissions. Proc. Intersoc. Energy Conversion Eng. Conf. 1997, 30, 944-948.
20. Ishida, M, personal communication
21. Mattisson T, Lyngfelt A and Cho P The use of iron oxide as an oxygen carrier in chemical-looping combustion of methane with inherent separation of CO2. submitted for publication, 2000.
22. Mattisson T, Lyngfelt A and Cho P, Possibility of using iron oxide as an oxygen carrier for combustion of methane with removal of CO2 - Application of chemical-looping combustion. To be presented at the Fifth International Conference on Greenhouse Gas Control Technologies, Cairns, Australia, 13th-16th August 2000.